

Immuno Oncology for Animals
Global IO and next generation animal health company for horses, cats and dogs
ANIMO stands for animals and IO … immuno-oncology with add on, advanced technologies – we are also developing new, breakthrough vaccines for horses and camels
We are an EDC, an ethics driven company
Our dual approach is on breakthrough products and generating solid returns for investors (high X-factors) at low risks and costs vs. far higher industry cost standards. For our investors and … horses !

77
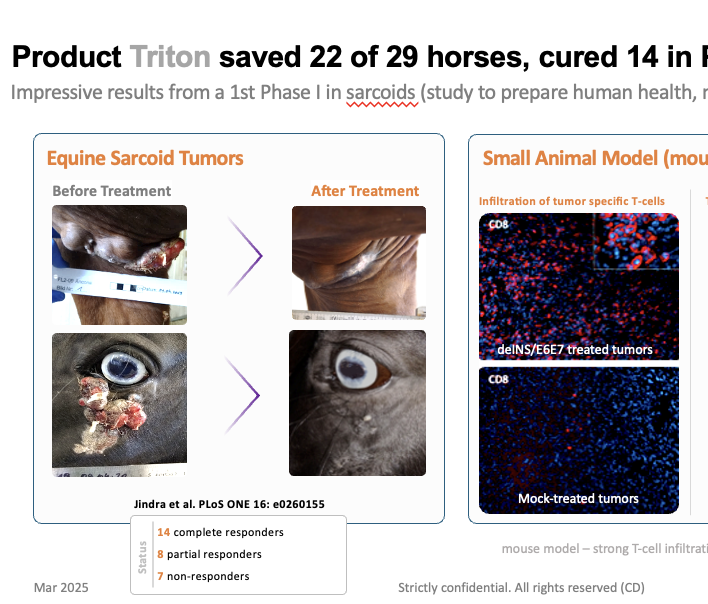
horses successfully treated on skin cancers (sarcoids). We need GMP-CMC production and one pivotal trial for EMA, USDA / FDA, APAC

27
years of university R&D and 435 man years of scientific work, university hospitals, leading in Europe and US

3
Peer reviewed publications on most impressive efficacy & safety data “similar to cure” (slight temperature increases, no other signals, 3 yrs of observation, most major IO cancer parameters covered)

– 2/3
“or more” of cost reduction due to AI and ANIMO mgmt. vs. industry R&D standards (AI driven, SWISSEXEL.COM)

Immuno – Oncology for Horses:
From Product to Global Profitability in Horses
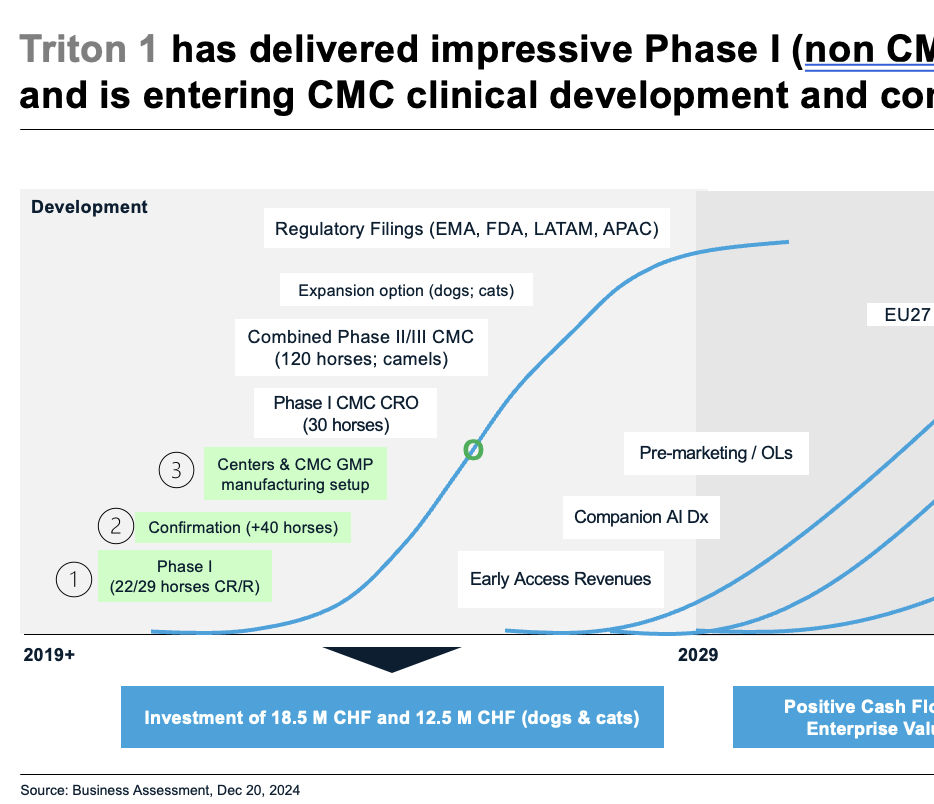
We follow our “roadmap to EMA & USDA (FDA) approval”, are prepared for global launches in 3-4 years. We presented at leading CEO & investor conferences in 2025 attracting interest and resonance by investors

Dual mechanism of action, vaccination and intra cellular stimulation, targeted cell death

Impressive CR/R and “cure” results

CMC and pivotal studies (II-III) “good to go”

Focus on clinical & pre-commercialisation in very few years (“hand in hand”, globally)
3-4
YEARS
TO GLOBAL REVENUES

We are an “execution company”
“When the Strategy is clear, Execution becomes the new strategy”
Peter Drucker (1965)
Our product is now entering clinical confirmation studies and will reach markets in 3-4 years as to plans. More than 78+ horses were successfully treated on sarcoids (skin cancers) including most severe cases in late stage (pre-metastatic bone disease). Our pivotal trials need “some more” actively treated horses and 1/3 of placebos (“nothing more”)

ADVANCED, TARGETED IO, INTRA CELLULAR ACTIVITY, UNIQUE IP
Science from leading university hospitals in Europe & US (27 years)
LATE STAGE R&D, LAUNCHES
Defined “roadmap” from “product to profitability”
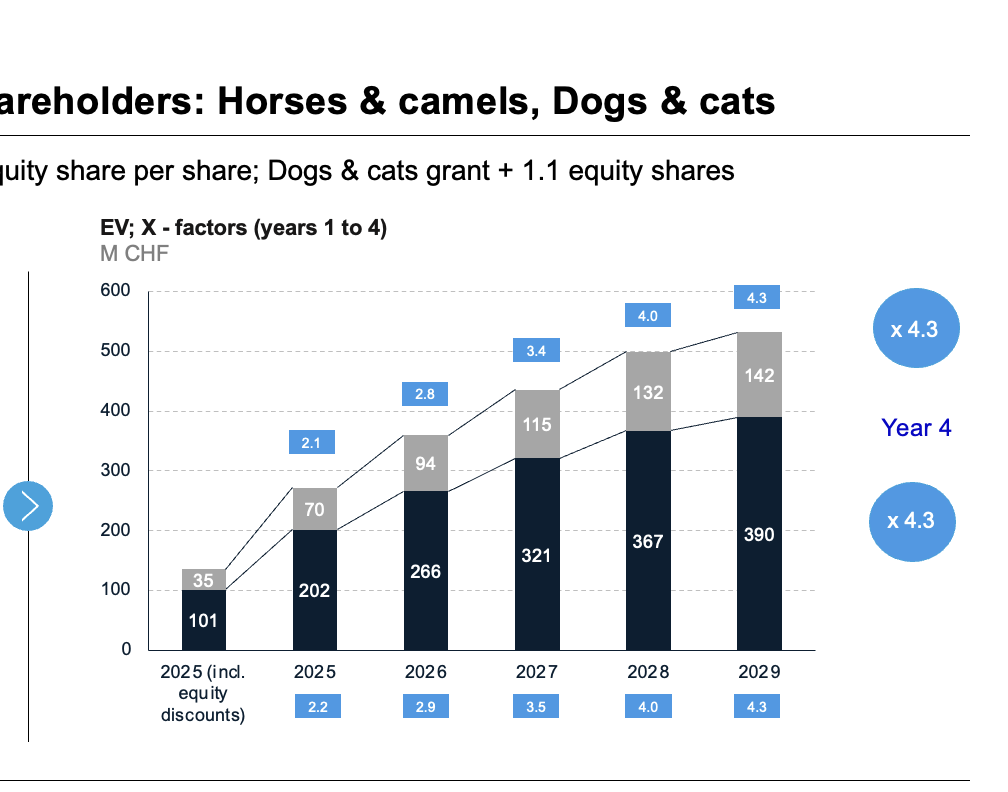
EBITA, EV, ROI & >4 X FACTORS
Expansion possible to build on first products

EDC – Ethics driven companies
We provide transparency and constant financial updates
As part of EDC companies, we were selected for our honest approach and developed by NEMOO AI Value Builders EDC AG. Our business plans are our financial investment plans. They are identical, externally, internally. We provide measurements at least 6-monthly, ad hoc to investors (in line with US-GAAP). We benefit from NEMOO AI EDCs support, experience, coaching, structures and network. We drive valuation, develop projects, people, teams, in a lean way with simple AI solutions – together. We also sit together for future options and expansion, opportunities arising. We hence, are learning & expand together

Experience
Our team is solid, well educated, science & ethics driven, R&D focused, strong in global commercial and finance (M&A, costs)

Capital
We partner with investors closely to create optimal value, developments and/or exits together

For Further Information
Our chosen finance, support & governing company NEMOO AI Value Builders AG is helping you to answer questions or share more as time evolves or need might be (NDA driven, fairly transparent)

Execution. It is our game.
From Product to Profitability
We follow a well defined, costs & risk mitigated synopsis, clinical protocol and financial plan (15 years to 2041). Developed with leading university clinics, PI and OLs. For finance, we follow a PE approach from Harvard, MIT, Stanford PE methodologies, private investor needs & their principles

–> Medical Confirmation
We successfully completed 3 yrs of clinical testing in 78+ horses, assured CMC GMP manufacturing for EMA, USDA/FDA, Middle East and APAC (pivotal trials)

–> Pivotal trials with leading centers
The last 2 studies are to start 2025/26, low costs and risks. We operate through 5 leading equine universities (Germany, Spain, US, UK, Abu Dhabi / Emirates) led by Germany & Switzerland (for Swiss Patent Box)

Launches
–> Study approvals ex ante (EMA, FDA)
Regulatory paths to be implemented with EMA (Paul Ehrlich Institute)
and USDA FDA, Emirates
MANAGEMENT & SUPERVISORY BOARD
Experienced Team with
Hands On Attitude
Our SWISS ANNIMO Health EDC Team consists of various people, experiences, backgrounds, operational track records in commercialisation and drug development (PAREXEL), manufacturing (LONZA), biotech and pharma (Roche, Novartis, AstraZeneca, Biogen, Roche Diagnostics), scientific and clinical development (BlueSky, Cubist, PAREXEL), rare diseases (SOBI), regulatory and finance (PAREXEL, LONZA, Roche, SP / Merck & Co.) & IO immunology / biotechnology (GreenHills, BlueSky, SOBI)

Finance, Global Taxation
Advisor IA
(ex SP/MSD,
Switzerland, USA)
Patents, IP & Legal
Advisor IA
(ex Amgen IP & Legal
Head EMEA, Switzerland)
Medical Strategies
Advisor IA
(Prof. of Kellogg MBA, Germany, Sweden)
People & Culture
Advisor IA
(ex MSD, Novartis, Swiss Investment Banking)
For investors, external partners, doctors and clinical centers

We are posting updates and relevant news from time to time on the development and science of our products in horses and camels
All data and business case reserved & protected by the authors:
Thank you for your interest
Claudia (16) & Christian


